Global Clinical Trial Management Software Market size is expected to grow from USD 1.4 Billion in 2022 to USD 4.02 Billion by 2030, at a CAGR of 14.1% during the forecast period (2023-2030).
Market Overview:
The Clinical Trial Management Software (CTMS) market is a vital component of the healthcare and life sciences industry, providing organizations with efficient tools to plan, manage, and monitor clinical trials. Clinical trials are fundamental in the development of new drugs, therapies, and medical devices. CTMS software streamlines the complex process of clinical trial management by centralizing data, automating workflows, and ensuring regulatory compliance.
Introspective Market Research provide comprehensive market research studies, delivering valuable insights and strategic guidance to businesses worldwide. Our operations are driven by accurate analysis and extensive coverage of all the areas to offer reliable reports.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
List of Prominent Players in the Clinical Trial Management Software Market:
Merge Healthcare Incorporated (U.S.),Bio-Optronics (U.S.),DSG INC (U.S.),ERT Clinical Bioclinica (U.S.),Oracle Corporation (U.S.),Medidata Solutions (U.S.),DATATRAK International, Inc. (U.S.),MedNet Solutions, Inc., (U.S.),Parexel International (U.S.),IBM (U.S.),ArisGlobal (U.S.) ,Veeva Systems (U.S.),MasterControl (U.S.),DZS Software Solutions (U.S.),RealTime Software Solutions LLC (U.S.),Advarra Technology Solutions (U.S.),Calyx (U.S.),Crucial Data Solutions (U.S.),ICON plc (Ireland),Ennov (France), and Other Major Players.
https://diamonddexch.com/
Realtime A.I. coaching and AI-driven learning as well as development simulations, businesses and institutions of higher education are changing the way knowledge is acquired and utilized. The latest technologies provide customized, effective and flexible learning solutions that are tailored to meet the specific demands of businesses as well as individuals.
Visit our website for more info https://www.expediteiot.co...
Contact us on +966502104086
To know more visit https://bit.ly/3hBp8yI
#lastmiledeliverymanagementsoftware #parcelpickupanddeliverysoftware #logisticsdeliverysoftware #AppDevelopment #OnDemandApps #iosappdevelopment #androidappdevelopment #MobileAppDevelopment #WebAppDevelopment #androidappdevelopmentcompany #startup #mobileappdevelopmentcompany #ondemandappdevelopment #sme #businessowner
Optical microscopes remain indispensable instruments in biological research, materials science, and clinical diagnostics due to their highresolution imaging, versatility, and relatively low cost compared with electron microscopy. These instruments employ visible light and glass lenses to magnify samples, enabling brightfield, darkfield, phasecontrast, and fluorescence techniques that reveal cellular structures, surface topography, and livecell dynamics.
Recent advances in optics, digital imaging, and software‐driven automation have expanded the market scope, allowing seamless integration with AI for realtime analysis. Laboratories benefit from faster workflows, improved image clarity, and minimized downtime, driving business growth across pharmaceutical R&D, semiconductor inspection, and academic institutions.
The global optical microscopes market is estimated to be valued at USD 2,906.1 Mn in 2025 and is expected to reach USD 4,266.9 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.64% from 2025 to 2032.
Optical Microscopes Market
https://www.coherentmarket...
Get More Insights On Optical Microscopes Market
https://justpaste.it/g0nfd
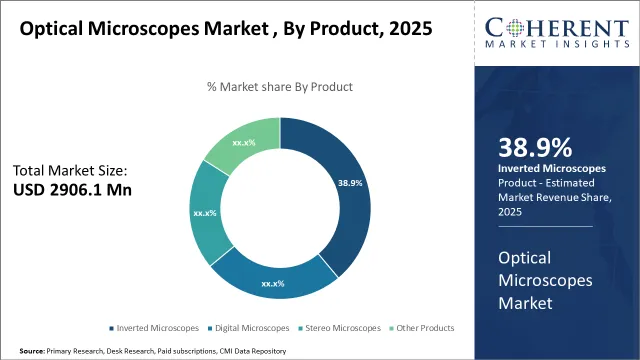
Optical Microscopes Market Size, Share & Analysis, 2025-2032
Optical Microscopes Market valuation is estimated to reach USD 2,906.1 Mn in 2025 and is anticipated to grow USD 4,266.9 Mn by 2032 with steady CAGR of 5.64%.
https://www.coherentmarketinsights.com/market-insight/optical-microscopes-market-5606Main Site - https://www.timesofisrael....
https://www.mid-day.com/li...
https://www.tribuneindia.c...
https://www.realtimeindia....
https://www.facebook.com/D...
https://www.facebook.com/D...
https://destiny-keto-acv.j...
https://destiny-keto-gummi...
https://destiny-keto-acv-r...
@destinyacvketous /destiny-keto-gummies-reviews-ingredients-natural-best-offer-reviews-urgent-customer-scam-warning-b" target="_blank" class="inline-link">https://medium.com/destin...
Dwngo social network website
Dwngo – The Social Media Platform! * Share your thoughts & ideas * Publish blogs & trending stories * Connect, engage & grow your networkJoin now & be part of the future of social networking! #SocialMedia #Blogging #Dwngo --https://dwngo.com/
"Data Bridge Market research has recently published the comprehensive business research on Asia-Pacific Eclinical Solutions Market includes historic data, present market trends, future product environment, marketing strategies, technological innovation, upcoming technologies, emerging trends or opportunities, and the technical progress in the related industry. Asia-Pacific Eclinical Solutions Market analysis report covers detailed value chain analysis of the market.
Asia-Pacific Eclinical Solutions Market business report provides exact information about market trends, industrial changes, and consumer behaviour etc. The report assists in outlining brand awareness, market landscape, possible future issues, industry trends and customer behaviour about industry which eventually leads to advanced business strategies. Being a verified and reliable source of information, this market research report offers a telescopic view of the existing market trends, emerging products, situations and opportunities that drives the business in the right direction of success. The Asia-Pacific Eclinical Solutions Market business report has been framed with the proper use of tools like SWOT analysis and Porter’s Five Forces analysis methods.
Access Full 350 Pages PDF Report @
https://www.databridgemark...
Data Bridge Market Research analyzes that the Asia-Pacific e-clinical solutions market is expected to reach a value of USD 2,867.92 million by 2030, at a CAGR of 12.9% during the forecast period. This market report also covers pricing analysis and technological advancements in depth.
Some of the major market players operating in the Asia-Pacific e-clinical solutions market are Oracle, Signant Health, MaxisIT, Paraxel International Corporation, Dassault Systemes, Clario, Mednet, OpenClinica, LLC, 4G Clinical, Veeva Systems, Saama Technologies, LLC, Anju, Castor, Medrio, Inc., ArisGlobal, Merative, Advarra, eClinical Solutions, LLC, Y-Prime LLC, and RealTime Software Solutions LLC, among others.
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the Asia-Pacific Eclinical Solutions Market.
Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches
Pediatric hearing aids are specialized electroacoustic devices designed to improve auditory perception in children with varying degrees of hearing impairment. These devices feature miniaturized processors, adaptive noise reduction, feedback cancellation, and Bluetooth connectivity, ensuring clear sound quality and seamless integration with educational technology. With ergonomic designs and childfriendly aesthetics, pediatric hearing aids address the unique anatomical and lifestyle requirements of young users, making them more comfortable and socially acceptable.
Early intervention through advanced hearing solutions not only facilitates speech and language development but also supports cognitive growth and academic performance. As awareness of congenital and acquired hearing loss increases, demand has surged for products that offer realtime monitoring, remote fitting, and teleaudiology services
The Global Pediatric Hearing Aid Market is estimated to be valued at USD 2.16 Bn in 2025 and is expected to reach USD 3.30 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.25% from 2025 to 2032.
Pediatric Hearing Aid Market
https://www.coherentmarket...
Get More Insights On Pediatric Hearing Aid Market
https://justpaste.it/gwdh4
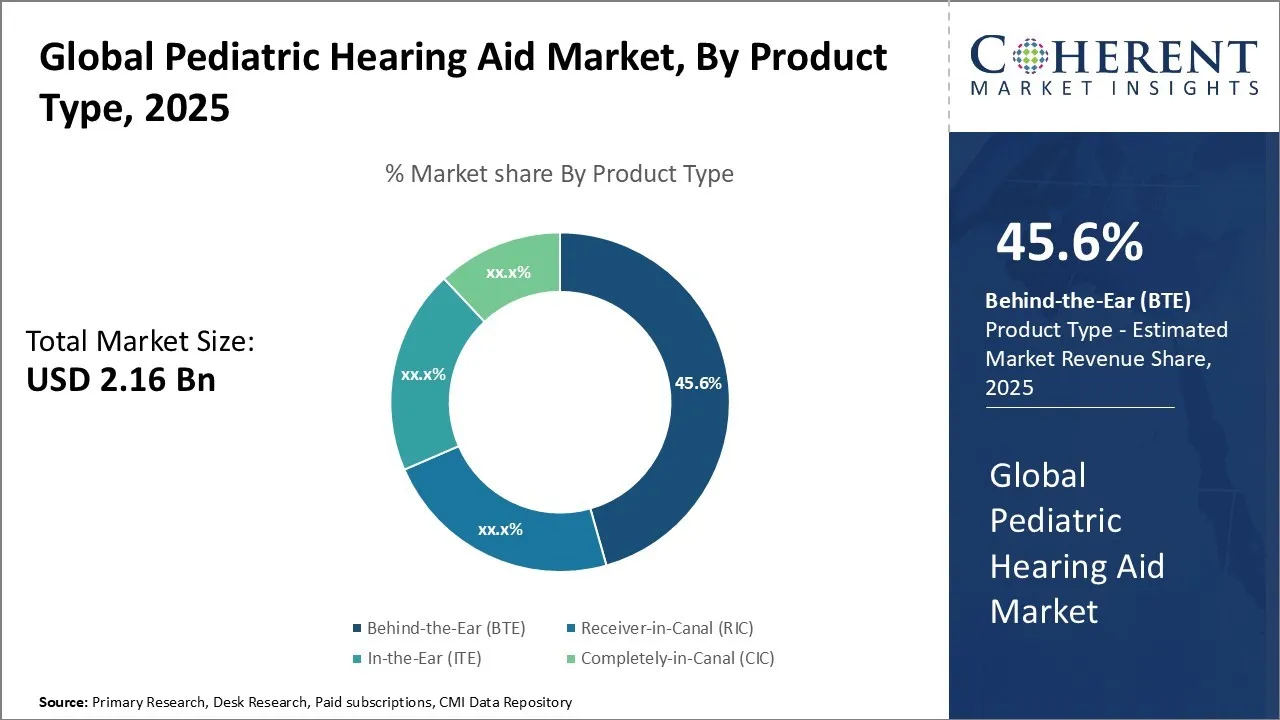
Pediatric Hearing Aid Market Share & Opportunities 2025-2032
Pediatric Hearing Aid Market valued at USD 2.16 Bn in 2025, is anticipated to reaching USD 3.30 Bn by 2032, with a steady annual growth rate of 6.25%.
https://www.coherentmarketinsights.com/industry-reports/pediatric-hearing-aid-markethttps://www.maximizemarket...
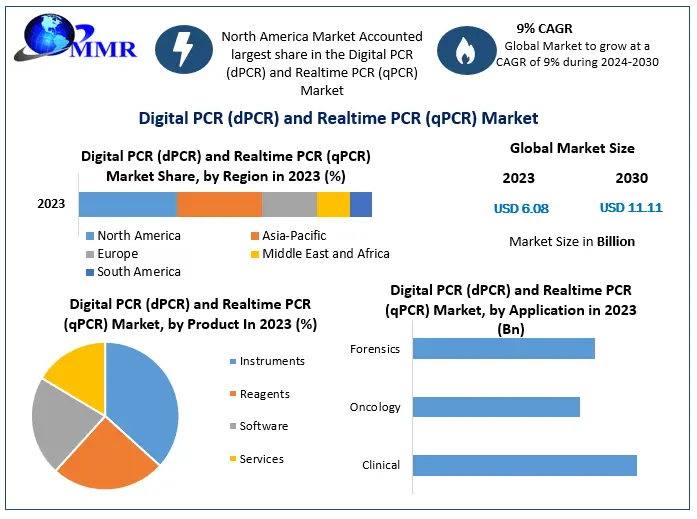
Digital PCR (dPCR) and Realtime PCR (qPCR) Market-Analysis
Digital PCR (dPCR) and Real-time PCR (qPCR) Market size is expected to grow at a CAGR of 9% during forecast period
https://www.maximizemarketresearch.com/market-report/global-digital-pcr-dpcr-and-real-time-pcr-qpcr-market/32992/Global Clinical Trial Management Software Market size is expected to grow from USD 1.4 Billion in 2022 to USD 4.02 Billion by 2030, at a CAGR of 14.1% during the forecast period (2023-2030).
Introspective Market Research provide comprehensive market research studies, delivering valuable insights and strategic guidance to businesses worldwide. Our operations are driven by accurate analysis and extensive coverage of all the areas to offer reliable reports.
The future demand and key players in the Clinical Trial Management Software market are poised to play pivotal roles in shaping the industry’s trajectory. Anticipated demand in the coming years is expected to be driven by specific factors, such as technological advancements, changing consumer behaviors, regulatory shifts, or global trends. As the market evolves, several key players will likely emerge as influential forces. Among the top contenders are leading companies or organizations, known for their innovation, market presence, and strategic initiatives.
Clinical trials have shifted from a paper-based to an electronic format. This increased use of cloud-based platforms is primarily due to the ease of compliance, privacy, data ownership, and auditing they provide for businesses. IT departments can eliminate unnecessary expenses by removing extensive, complex infrastructure and deploying cloud solutions, primarily hosted or supported by external vendors.
List Of Prominent Players In The Clinical Trial Management Software Market:
Merge Healthcare Incorporated (U.S.),Bio-Optronics (U.S.),DSG INC (U.S.),ERT Clinical Bioclinica (U.S.),Oracle Corporation (U.S.),Medidata Solutions (U.S.),DATATRAK International, Inc. (U.S.),MedNet Solutions, Inc., (U.S.),Parexel International (U.S.),IBM (U.S.),ArisGlobal (U.S.) ,Veeva Systems (U.S.),MasterControl (U.S.),DZS Software Solutions (U.S.),RealTime Software Solutions LLC (U.S.),Advarra Technology Solutions (U.S.),Calyx (U.S.),Crucial Data Solutions (U.S.),ICON plc (Ireland),Ennov (France), and Other Major Players.
Request Free Sample Copy (To Understand the Complete Structure of this Report [Summary + TOC]
https://introspectivemarke...
In today's globalized world, the transportation and storage of temperaturesensitive products have become increasingly complex. From food and pharmaceuticals to chemicals and electronics, maintaining the integrity of these products throughout the supply chain is crucial. Time Temperature Indicator (TTI) labels have emerged as a gamechanging solution to ensure product safety and quality. These innovative labels provide realtime monitoring of temperature exposure, enabling manufacturers, distributors, and consumers to make informed decisions about the usability and safety of their products.
Time Temperature Indicator labels are smart labels that visually indicate the cumulative timetemperature history of a product. These labels contain a temperaturesensitive material that reacts to heat exposure over time, causing a visible change in color or appearance. The change in the label's appearance serves as a clear and easytointerpret indicator of whether the product has been exposed to temperatures outside its acceptable range and for how long.
Get More Insights On Time Temperature Indicator Labels
https://www.zupyak.com/p/4...
#TimeTemperatureIndicators , #ColdChain , #ColdChainManagement ,
#SupplyChainInnovation , #SmartPackaging , #LogisticsTechnology

Time Temperature Indicator Labels Revolutionizing Cold Chain Safety Globally | Zupyak
https://www.zupyak.com/p/4564697/t/time-temperature-indicator-labels-revolutionizing-cold-chain-safety-globally
Global Clinical Trial Management Software Market size is expected to grow from USD 1.4 Billion in 2022 to USD 4.02 Billion by 2030, at a CAGR of 14.1% during the forecast period (2023-2030).
Clinical Trial Management Systems (CTMS) facilitate the planning, execution, and monitoring of clinical trials, streamlining processes and ensuring regulatory compliance. These systems integrate various functionalities, including participant recruitment, data management, and reporting, enhancing efficiency and accuracy in clinical research. The CTMS Market is driven by technological advancements, increasing clinical trial complexities, and regulatory requirements, shaping the landscape of clinical research.
Major Companies and Competitive Landscape:
Merge Healthcare Incorporated (U.S.),Bio-Optronics (U.S.),DSG INC (U.S.),ERT Clinical Bioclinica (U.S.),Oracle Corporation (U.S.),Medidata Solutions (U.S.),DATATRAK International, Inc. (U.S.),MedNet Solutions, Inc., (U.S.),Parexel International (U.S.),IBM (U.S.),ArisGlobal (U.S.) ,Veeva Systems (U.S.),MasterControl (U.S.),DZS Software Solutions (U.S.),RealTime Software Solutions LLC (U.S.),Advarra Technology Solutions (U.S.),Calyx (U.S.),Crucial Data Solutions (U.S.),ICON plc (Ireland),Ennov (France), and Other Major Players.
Request Sample Pages of this Research Study at –
https://introspectivemarke...
Market Driver:
A key market driver is the rising number and complexity of clinical trials. The pharmaceutical and biotechnology industries are experiencing an upsurge in the volume and intricacy of clinical trials, driven by advances in medical research, the pursuit of personalized medicine, and the need for novel therapies. The demand for efficient and centralized management of diverse trial processes, including patient recruitment, data collection, and regulatory compliance, propels the adoption of CTMS. As the industry strives for more robust, transparent, and data-driven clinical trials, the use of CTMS becomes imperative to navigate the intricacies of modern trial management.
Market Opportunity:
An emerging market opportunity lies in the integration of artificial intelligence (AI) and machine learning (ML) capabilities within CTMS. By leveraging these technologies, CTMS can offer enhanced predictive analytics, data-driven insights, and automation of routine tasks. This pr
Realtime biometric machine make it easy to keep track of when employees are present. It works with both fingerprint and face recognition, gives correct real-time data, and is perfect for factories, schools, and offices that need to track attendance automatically.
Buy now: https://shop.sbj-store.com...
Global Clinical Trial Management Software Market size is expected to grow from USD 1.4 Billion in 2022 to USD 4.02 Billion by 2030, at a CAGR of 14.1% during the forecast period (2023-2030)
Market Overview:
The Clinical Trial Management Software (CTMS) market is a vital component of the healthcare and life sciences industry, providing organizations with efficient tools to plan, manage, and monitor clinical trials. Clinical trials are fundamental in the development of new drugs, therapies, and medical devices. CTMS software streamlines the complex process of clinical trial management by centralizing data, automating workflows, and ensuring regulatory compliance.
Introspective Market Research provide comprehensive market research studies, delivering valuable insights and strategic guidance to businesses worldwide. Our operations are driven by accurate analysis and extensive coverage of all the areas to offer reliable reports.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
List of Prominent Players in the Clinical Trial Management Software Market:
Merge Healthcare Incorporated (U.S.),Bio-Optronics (U.S.),DSG INC (U.S.),ERT Clinical Bioclinica (U.S.),Oracle Corporation (U.S.),Medidata Solutions (U.S.),DATATRAK International, Inc. (U.S.),MedNet Solutions, Inc., (U.S.),Parexel International (U.S.),IBM (U.S.),ArisGlobal (U.S.) ,Veeva Systems (U.S.),MasterControl (U.S.),DZS Software Solutions (U.S.),RealTime Software Solutions LLC (U.S.),Advarra Technology Solutions (U.S.),Calyx (U.S.),Crucial Data Solutions (U.S.),ICON plc (Ireland),Ennov (France), and Other Major Players.
Request PDF Sample Copy of Report: (Including
Ervaar gemoedsrust met realtime detectie van bedreigingen en directe waarschuwingen. Blijf cybercriminelen een stap voor met automatische updates die de nieuwste beveiligingsprotocollen bieden. U hoeft zich geen zorgen meer te maken over identiteitsdiefstal of datalekken. Onze uitgebreide bescherming omvat al uw apparaten, van pc's tot smartphones, waardoor u naadloze beveiliging in uw hele digitale leven krijgt. https://privacyenbeschermi...