Adalimumab Biosimilar Market Latest Trend, Share Analysis, Growth, and Application Forecast 2024 - 2031
The "Adalimumab Biosimilar Market" is a dynamic and rapidly evolving sector, with significant advancements and growth anticipated by 2031. Comprehensive market research reveals a detailed analysis of market size, share, and trends, providing valuable insights into its expansion. This report delves into segmentation and definition, offering a clear understanding of market components and drivers. Employing SWOT and PESTEL analyses, the study evaluates the market's strengths, weaknesses, opportunities, and threats, alongside political, economic, social, technological, environmental, and legal factors. Expert opinions and recent developments highlight the geographical distribution and forecast the market's trajectory, ensuring a robust foundation for strategic planning and investment.
What is the projected market size & growth rate of the Adalimumab Biosimilar Market?
Market Analysis and Size
According to GLOBOCAN 2020, there will be 2,281,658 new cancer cases and nearly 612,390 deaths in the United States in 2020. Furthermore, key market participants predicted market growth over the forecast period through various strategic activities such as product launches, mergers, and acquisitions. For instance, in July 2021, the FDA approved Viatris Inc.'s (formerly Mylan Pharmaceuticals Inc.) SEMGLEE (insulin-glargine-yfgn), a biosimilar to LANTUS (insulin glargine).
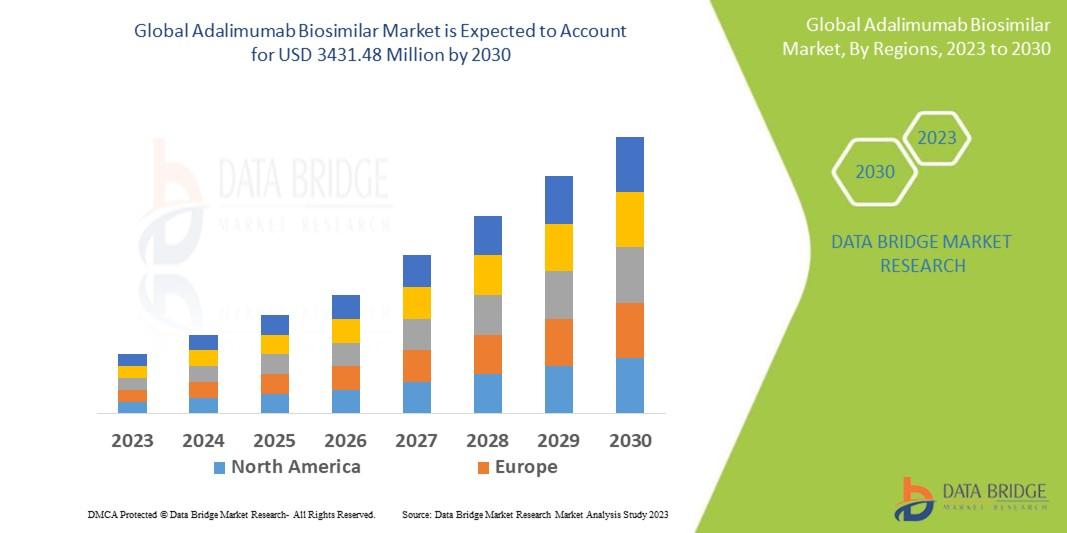
Data Bridge Market Research analyses that the adalimumab biosimilar market which is USD 598.30 million in 2022, is expected to reach USD 3431.48 million by 2030, at a CAGR of 24.4% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Browse Detailed TOC, Tables and Figures with Charts which is spread across 350 Pages that provides exclusive data, information, vital statistics, trends, and competitive landscape details in this niche sector.
This research report is the result of an extensive primary and secondary research effort into the Adalimumab Biosimilar market. It provides a thorough overview of the market's current and future objectives, along with a competitive analysis of the industry, broken down by application, type and regional trends. It also provides a dashboard overview of the past and present performance of leading companies. A variety of methodologies and analyses are used in the research to ensure accurate and comprehensive information about the Adalimumab Biosimilar Market.
Get a Sample PDF of Report - https://www.databridgemark...
Which are the driving factors of the Adalimumab
The "Adalimumab Biosimilar Market" is a dynamic and rapidly evolving sector, with significant advancements and growth anticipated by 2031. Comprehensive market research reveals a detailed analysis of market size, share, and trends, providing valuable insights into its expansion. This report delves into segmentation and definition, offering a clear understanding of market components and drivers. Employing SWOT and PESTEL analyses, the study evaluates the market's strengths, weaknesses, opportunities, and threats, alongside political, economic, social, technological, environmental, and legal factors. Expert opinions and recent developments highlight the geographical distribution and forecast the market's trajectory, ensuring a robust foundation for strategic planning and investment.
What is the projected market size & growth rate of the Adalimumab Biosimilar Market?
Market Analysis and Size
According to GLOBOCAN 2020, there will be 2,281,658 new cancer cases and nearly 612,390 deaths in the United States in 2020. Furthermore, key market participants predicted market growth over the forecast period through various strategic activities such as product launches, mergers, and acquisitions. For instance, in July 2021, the FDA approved Viatris Inc.'s (formerly Mylan Pharmaceuticals Inc.) SEMGLEE (insulin-glargine-yfgn), a biosimilar to LANTUS (insulin glargine).
Data Bridge Market Research analyses that the adalimumab biosimilar market which is USD 598.30 million in 2022, is expected to reach USD 3431.48 million by 2030, at a CAGR of 24.4% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Browse Detailed TOC, Tables and Figures with Charts which is spread across 350 Pages that provides exclusive data, information, vital statistics, trends, and competitive landscape details in this niche sector.
This research report is the result of an extensive primary and secondary research effort into the Adalimumab Biosimilar market. It provides a thorough overview of the market's current and future objectives, along with a competitive analysis of the industry, broken down by application, type and regional trends. It also provides a dashboard overview of the past and present performance of leading companies. A variety of methodologies and analyses are used in the research to ensure accurate and comprehensive information about the Adalimumab Biosimilar Market.
Get a Sample PDF of Report - https://www.databridgemark...
Which are the driving factors of the Adalimumab
03:06 PM - Jun 07, 2024 (UTC)